Associação Portuguesa de Investigação em Cancro
Hereditary Diffuse Gastric Cancer Syndrome CDH1 Mutations and Beyond
Hereditary Diffuse Gastric Cancer Syndrome CDH1 Mutations and Beyond
Authors and Affiliations:
Samantha Hansford, MSc1,2; Pardeep Kaurah, MSc3,5; Hector Li-Chang, MD1,2; Michelle Woo, PhD1,2; Janine Senz, BSc1,2; Hugo Pinheiro, PhD4; Kasmintan A. Schrader, MBBS, PhD5; David F. Schaeffer, MD6; Karey Shumansky, MSc7; George Zogopoulos, MD8,9; Teresa Almeida Santos, MD, PhD10,11; Isabel Claro, MD12; Joana Carvalho, PhD4; Cydney Nielsen, PhD2,7; Sarah Padilla, BSc1; Amy Lum, BSc1; Aline Talhouk, PhD1,2; Katie Baker-Lange, MSc13; Sue Richardson, RGN14; Ivy Lewis, BN, RN15; Noralane M. Lindor, MD16; Erin Pennell, RN17; Andree MacMillan, MSc15; Bridget Fernandez, MD15; Gisella Keller, PhD18; Henry Lynch, MD19; Sohrab P. Shah, PhD7; Parry Guilford, MSc, PhD20; Steven Gallinger, MD21,22; Giovanni Corso, MD, PhD23,24; Franco Roviello, MD24,25; Carlos Caldas, MD14; Carla Oliveira, PhD4,26; Paul D. P. Pharoah, PhD27,28; David G. Huntsman, MD1,2,7,29
2Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, British Columbia, Canada
3Department of Medical Genetics, University of British Columbia, Vancouver, British Columbia, Canada
4Expression Regulation in Cancer Group, IPATIMUP–Institute of Molecular Pathology and Immunology of the University of Porto, Porto, Portugal
5Hereditary Cancer Program, BC Cancer Agency, Vancouver, British Columbia, Canada
6Division of Anatomical Pathology, Vancouver General Hospital, University of British Columbia, Vancouver, British Columbia, Canada
7Department of Molecular Oncology, BC Cancer Agency, Vancouver, British Columbia, Canada
8The Research Institute of the McGill University Health Centre, Montreal, Quebec, Canada
9Rosalind and Morris Goodman Cancer Research Centre, Montreal, Quebec, Canada
10Human Reproduction Service, University Hospitals of Coimbra, Coimbra, Portugal
11Faculty of Medicine, University of Coimbra, Coimbra, Portugal
12Instituto Português de Oncologia de Lisboa Francisco Gentil E.P.E., Lisbon Portugal
13Frauenshuh Cancer Center, Park Nicollet Clinic, St Louis Park, Minnesota
14Cancer Research UK Cambridge Institute, Cambridge, England
15Provincial Medical Genetics Program, St John’s, Newfoundland, Canada
16Department of Health Science Research, Mayo Clinic, Scottsdale, Arizona
17Cancer Care Program, Dr. H. Bliss Murphy Cancer Centre Eastern Health, St John’s, Newfoundland, Canada
18Institute of Pathology, Technische Universität München, München, Germany
19Creighton’s Hereditary Cancer Center, Omaha, Nebraska
20Cancer Genetics Laboratory, Department of Biochemistry, University of Otago, Dunedin, New Zealand
21Division of General Surgery, Department of Surgery, University of Toronto, Toronto, Ontario, Canada
22Samuel Lunenfeld Research Institute, Mount Sinai Hospital Toronto, Ontario, Canada
23Department of Experimental Oncology, European Institute of Oncology, Milano, Italy
24Department of Medical Surgical Sciences and Neurosciences, Section of General Surgery and Surgical Oncology, University of Siena, Siena, Italy
25Istituto Toscano Tumori (ITT), University Hospital of Siena, Siena, Italy
26Faculty of Medicine, University of Porto, Porto, Portugal
27Department of Oncology, University of Cambridge, Strangeway’s Research Laboratory, Wort’s Causeway, Cambridge, England
28Department of Public Health and Primary Care, University of Cambridge, Strangeway’s Research Laboratory, Wort’s Causeway, Cambridge, England
29Department of Obstetrics and Gynecology (Huntsman), University of British Columbia, Vancouver, British Columbia, Canada
Abstract:
Importance E-cadherin (CDH1) is a cancer predisposition gene mutated in families meeting clinically defined hereditary diffuse gastric cancer (HDGC). Reliable estimates of cancer risk and spectrum in germline mutation carriers are essential for management. For families without CDH1 mutations, genetic-based risk stratification has not been possible, resulting in limited clinical options.
Objectives To derive accurate estimates of gastric and breast cancer risks in CDH1 mutation carriers and determine if germline mutations in other genes are associated with HDGC.
Design, Setting, and Participants Testing for CDH1 germline mutations was performed on 183 index cases meeting clinical criteria for HDGC. Penetrance was derived from 75 mutation-positive families from within this and other cohorts, comprising 3858 probands (353 with gastric cancer and 89 with breast cancer). Germline DNA from 144 HDGC probands lacking CDH1 mutations was screened using multiplexed targeted sequencing for 55 cancer-associated genes.
Main Outcomes and Measures Accurate estimates of gastric and breast cancer risks in CDH1 mutation carriers and the relative contribution of other cancer predisposition genes in familial gastric cancers.
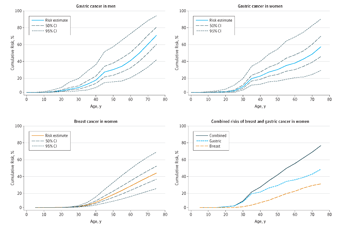
Results Thirty-one distinct pathogenic CDH1 mutations (14 novel) were identified in 34 of 183 index cases (19%). By the age of 80 years, the cumulative incidence of gastric cancer was 70% (95% CI, 59%-80%) for males and 56% (95% CI, 44%-69%) for females, and the risk of breast cancer for females was 42% (95% CI, 23%-68%). In CDH1 mutation–negative index cases, candidate mutations were identified in 16 of 144 probands (11%), including mutations within genes of high and moderate penetrance: CTNNA1, BRCA2, STK11, SDHB, PRSS1, ATM, MSR1, and PALB2.
Conclusions and Relevance This is the largest reported series of CDH1 mutation carriers, providing more precise estimates of age-associated risks of gastric and breast cancer that will improve counseling of unaffected carriers. In HDGC families lacking CDH1 mutations, testing of CTNNA1 and other tumor suppressor genes should be considered. Clinically defined HDGC families can harbor mutations in genes (ie, BRCA2) with different clinical ramifications from CDH1. Therefore, we propose that HDGC syndrome may be best defined by mutations in CDH1 and closely related genes, rather than through clinical criteria that capture families with heterogeneous susceptibility profiles.
Journal: JAMA Oncology
Link: http://oncology.jamanetwork.com/article.aspx?articleid=2108851






