Associação Portuguesa de Investigação em Cancro
Atomic force microscopy and graph analysis to study the P-cadherin/SFK mechanotransduction signalling in breast cancer cells
Atomic force microscopy and graph analysis to study the P-cadherin/SFK mechanotransduction signalling in breast cancer cells
AS Ribeiro1,2, FA Carvalho3, J Figueiredo1,2, R Carvalho1, T Mestre4, J Monteiro1, AF Guedes3, M Fonseca4, J Sanches4, R Seruca1,2,5, NC Santos3, J Paredes1,2,5
1 i3S - Instituto de Investigação e Inovação em Saúde, Universidade do Porto, Portugal;
2 Institute of Molecular Pathology and Immunology of the University of Porto;
3 Instituto de Medicina Molecular, Faculdade de Medicina, Universidade de Lisboa, Lisbon, Portugal;
4 IST-ISR, Lisbon, Portugal; 5Medical Faculty of the University of Porto, Porto, Portugal;
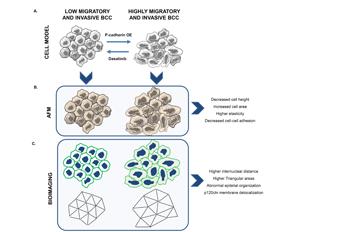
Physical forces mediated by cell-cell adhesion molecules, as cadherins, play a crucial role in preserving normal tissue architecture. Accordingly, altered cadherins’ expression has been documented as a common event during cancer progression. However, in most studies, no data exists linking pro-tumorigenic signaling and variations in the mechanical balance mediated by adhesive forces. In breast cancer, P-cadherin overexpression increases in vivo tumorigenic ability, as well as in vitro cell invasion, by activating Src family kinase (SFK) signalling. Though, it is not known how P-cadherin and SKF activation impact cell-cell biomechanical properties. In the present work, using atomic force microscopy (AFM) images, cell stiffness and cell-cell adhesion measurements, and undirected graph analysis based on microscopic images, we demonstrated that P-cadherin overexpression promotes significant alterations in cell’s morphology, by decreasing cellular height and increasing its area. It also affects biomechanical properties, by decreasing cell-cell adhesion and cell stiffness. Further, cellular network analysis showed alterations in intercellular organization, which associates with cell-cell adhesion dysfunction, destabilization of E-cadherin/p120ctn membrane complex and with increased cell invasion. Remarkably, inhibition of SFK signaling, using dasatinib, reverted the pathogenic P-cadherin induced effects, by increasing cell’s height, cell-cell adhesion and cell stiffness, and generating more compact epithelial aggregates, as quantified by intercellular network analysis. In conclusion, P-cadherin/SFK signalling induces topological, morphological and biomechanical cell-cell alterations which are associated to more invasive breast cancer cells. These effects could be further reverted by dasatinib treatment, demonstrating the applicability of AFM and cell network diagrams for measuring epithelia biomechanical properties and structural organization.
Journal: Nanoscale journal
http://pubs.rsc.org/en/Content/ArticleLanding/2016/NR/C6NR04465D#!divAbstract




