Associação Portuguesa de Investigação em Cancro
Recuperação da expressão da proteína Caderina-E normal, através de um tRNA-supressor, em células de cancro
Recuperação da expressão da proteína Caderina-E normal, através de um tRNA-supressor, em células de cancro
Este trabalho baseia-se na utilização de tRNAs-supressores como uma ferramenta terapêutica possível para síndromes de cancro hereditário, onde cerca de 10-20% das mutações descritas são mutações «nonsense». Um tRNA-supressor é um tRNA que pode ser alterado para reconhecer codões de paragem prematuros (mutações "nonsense"), promovendo a produção de proteínas de comprimento total. Como modelo utilizou-se o cancro gástrico difuso hereditário (HDGC), que está associado a mutações no gene CDH1, que codifica a molécula de adesão Caderina-E. Demonstramos, pela primeira vez, que o comprimento total e funcional da Caderina-E pode ser eficientemente recuperado em células de cancro gástrico a partir de um alelo mutante usando um supressor de tRNA. Mais importante, esta estratégia pode ser aplicada a outras doenças genéticas, sendo particularmente significativa para as síndromes de cancro hereditário. Outros estudos sobre este tipo de estratégia podem abrir novos caminhos para o tratamento do cancro.
Autores e afiliações:
Bordeira-Carriço R1, Ferreira D1, Mateus DD1, Pinheiro H1, Pêgo AP2, Santos MA3, Oliveira C4.
- 1Expression Regulation in Cancer Group, Institute of Molecular Pathology and Immunology of the University of Porto (IPATIMUP), Porto, Portugal.
- 21] INEB, Instituto de Engenharia Biomédica, Universidade do Porto, Porto, Portugal [2] Universidade do Porto-Instituto de Ciências Biomédicas Abel Salazar (ICBAS), Porto, Portugal [3] Universidade do Porto-Faculdade de Engenharia, Porto, Portugal.
- 3RNA Biology Laboratory, Department of Biology and CESAM, University of Aveiro, Aveiro, Portugal.
- 41] Expression Regulation in Cancer Group, Institute of Molecular Pathology and Immunology of the University of Porto (IPATIMUP), Porto, Portugal [2] Faculty of Medicine of the University of Porto, Porto, Portugal.
Abstract:
Hereditary diffuse gastric cancer (HDGC) syndrome, although rare, is highly penetrant at an early age, and is severe and incurable because of ineffective screening tools and therapy. Approximately 45% of HDGC families carry germline CDH1/E-cadherin alterations, 20% of which are nonsense leading to premature protein truncation. Prophylactic gastrectomy is the only recommended approach for all asymptomatic CDH1 mutation carriers. Suppressor-tRNAs can replace premature stop codons (PTCs) with a cognate amino acid, inducing readthrough and generating full-length proteins. The use of suppressor-tRNAs in HDGC patients could therefore constitute a less invasive therapeutic option for nonsense mutation carriers, delaying the development of gastric cancer. Our analysis revealed that 23/108 (21.3%) of E-cadherin-mutant families carried nonsense mutations that could be potentially corrected by eight suppressor-tRNAs, and arginine was the most frequently affected amino acid. Using site-directed mutagenesis, we developed an arginine suppressor-tRNA vector to correct one HDGC nonsense mutation. E-cadherin- deficient cell lines were transfected with plasmids carrying simultaneously the suppressor-tRNA and wild-type or mutant CDH1 mini-genes. RT-PCR, western blot, immunofluorescence, flow cytometry and proximity ligation assay (PLA) were used to establish the model, and monitor mRNA and protein expression and function recovery from CDH1 vectors. Cells expressing a CDH1 mini-gene, carrying a nonsense mutation and the suppressor-tRNA, recovered full-length E-cadherin expression and its correct localization and incorporation into the adhesion complex. This is the first demonstration of functional recovery of a mutated causative gene in hereditary cancer by cognate amino acid replacement with suppressor-tRNAs. Of the HDGC families, 21.3% are candidates for correction with suppressor-tRNAs to potentially delay cancer onset.European Journal of Human Genetics advance online publication, 15 January 2014; doi:10.1038/ejhg.2013.292.
Revista:
European Journal of Human Genetics
Link:
http://www.nature.com/ejhg/journal/vaop/ncurrent/full/ejhg2013292a.html
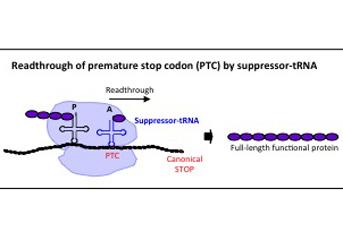
Legend of Figure 1:
During translation of the nonsense-mutated mRNA, the suppressor-tRNA, whose anticodon is mutated to recognize the premature stop codon (PTC), is able to insert the correct amino acid in the mutation site, leading to the production of a wild-type protein.




