Associação Portuguesa de Investigação em Cancro
Nanotecnologia inovadora para o silenciamento de proteínas envolvidas no cancro
Nanotecnologia inovadora para o silenciamento de proteínas envolvidas no cancro
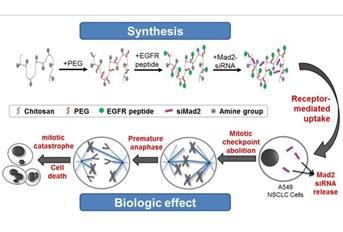
Trabalho de investigação de grupo português, em parceria com grupo da Northeastern University de Boston, Estados Unidos, permitiu desenvolver um sistema de base nanotecnológica, dirigida especificamente a células cancerígenas, com o objetivo de entregar fármacos com a capacidade de silenciar proteínas envolvidas na mitose celular (RNA de interferência) e reduzir em quase 100% os níveis de expressão da proteína. Esta estratégia terapêutica poderá resultar na morte seletiva de células cancerígenas, e em avanços significativos no tratamento do cancro.
Ana Vanessa Nascimento a,b, Amit Singh c, Hassan Bousbaa a,d, Domingos Ferreira b, Bruno Sarmento a,e, Mansoor M. Amiji c,
a CESPU, Instituto de Investigação e Formação Avançada em Ciências e Tecnologias da Saúde, IINFACTS, Rua Central de Gandra 1317, 4585-116 Gandra PRD, Portugal
b Laboratory of Pharmaceutical Technology, Faculty of Pharmacy, University of Porto, Portugal
c Department of Pharmaceutical Sciences, School of Pharmacy, Bouvé College of Health Sciences, Northeastern University, Boston 02115-5000, USA
d Center of Medicinal Chemistry of the University of Porto (CEQUIMED-UP), Portugal e INEB – Instituto de Engenharia Biomédica, Porto, Portugal
Abstract:
RNA interference (RNAi) has emerged as a powerful strategy in cancer therapy since it allows silencing of specific genes associated with tumor progression and resistance. Mad2 is an essential mitotic checkpoint component required for accurate chromosome segregation during mitosis and its complete abolition leads to cell death. We have developed an epidermal growth factor receptor (EGFR)-targeted chitosan system for silencing Mad2 gene as a strategy to efficiently induce cell death in EGFR overexpressing human A549 non-small cell lung cancer (NSCLC) cells. Control and EGFR-targeted chitosan nanoparticles loaded with small interfering RNAs (siRNAs) against Mad2 were formulated and characterized for size, charge, morphology, and encapsulation efficiency. Qualitative and quantitative intracellular uptake studies by confocal imaging and flow cytometry, respectively, showed time-dependent enhanced and selective intracellular internalization of EGFR-targeted nanoparticles compared to non-targeted system. Targeted nanoparticles showed near-complete depletion of Mad2 expression in A549 cells contrasting with the partial depletion in the non-targeted system. Accordingly, Mad2-silencing induced apoptotic cell death was confirmed by cytotoxicity assay and flow cytometry. Our results demonstrate that EGFR-targeted chitosan loaded with Mad2 siRNAs is a potent delivery system for selective killing of cancer cells.
Revista: Molecular Pharmaceutics
http://pubs.acs.org/doi/abs/10.1021/mp5002894




