Associação Portuguesa de Investigação em Cancro
Identificação de um novo alvo terapêutico contra a leucemia de linfócitos T
Identificação de um novo alvo terapêutico contra a leucemia de linfócitos T
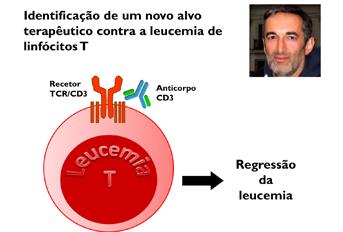
Com a importante colaboração de equipas de investigação francesas, o investigador Nuno Rodrigues dos Santos, atualmente afiliado no Instituto de Investigação e Inovação em Saúde da Universidade do Porto (i3S), descobriu que a estimulação de um recetor específico dos linfócitos T (TCR) levava à morte de células de leucemia de linfócitos T, uma doença maligna que afeta maioritariamente crianças e adolescentes, mas também adultos. Esta descoberta surgiu com o estudo de murganhos com leucemia, no Centro de Investigação em Biomedicina da Universidade do Algarve, onde se verificou que a estimulação do TCR através de um antigénio ou da inoculação de um anticorpo específico curava ou prolongava o tempo de vida dos murganhos com leucemia. Estes resultados foram posteriormente confirmados pelos colaboradores em França, tratando com anticorpo murganhos transplantados com leucemias humanas. Assim, estas descobertas demonstram que o tratamento com um anticorpo estimulador do TCR pode vir a ser uma nova terapia contra a leucemia de linfócitos T.
Amélie Trinquand1, Nuno R. dos Santos2,3, Christine Tran Quang3,4, Francesca Rocchetti3,4, Benedetta Zaniboni3,4, Mohamed Belhocine1,5, Cindy Da Costa de Jesus3,4, Ludovic Lhermitte1, Melania Tesio1, Michael Dussiot6, François-Loïc Cosset7, Els Verhoeyen7,8, Françoise Pflumio9, Norbert Ifrah10, Hervé Dombret11, Salvatore Spicuglia5, Lucienne Chatenoud12, David-Alexandre Gross12, Olivier Hermine 6,13, Elizabeth Macintyre1, Jacques Ghysdael3,4,* and Vahid Asnafi1,*
1 Université Paris Descartes Sorbonne Cité, Institut Necker-Enfants Malades (INEM), Institut national de recherche médicale (INSERM) U1151, and Laboratory of Onco-Hematology, Assistance Publique-Hôpitaux de Paris (AP-HP), Hôpital Necker Enfants-Malades, Paris, France.
2 Centre for Biomedical Research (CBMR), University of Algarve, Faro, Portugal.
3 Institut Curie, PSL Research University, CNRS UMR 3348, Orsay, France.
4 Université Paris Sud, Université Paris-Saclay, CNRS UMR 3348, Orsay, France.
5 Technological Advances for Genomics and Clinics (TAGC), INSERM U1090, Université de la Méditerranée, Marseille, France.
6 INSERM UMR 1163 and CNRS ERL 8654, Laboratory of Cellular and Molecular Mechanisms of Hematological Disorders and Therapeutic Implications, Laboratory of Excellence GR-Ex, Imagine Institute and Paris Descartes University, Sorbonne Paris Cité, Paris, France.
7 CIRI, EVIR Team, INSERM U1111, CNRS UMR 5308, Université de Lyon-1, ENS de Lyon, Lyon, France.
8 INSERM U1065, C3M, Equipe “Contrôle Métabolique des Morts Cellulaires,” Nice, France.
9 Laboratoire des Cellules Souches Hématopoïétiques et Leucémiques, UMR 967, INSERM, Commissariat à l'Energie Atomique, Université Paris Diderot, Université Paris 11, Institut de Radiobiologie Cellulaire et Moléculaire, équipe labellisée Ligue Nationale contre le Cancer, Fontenay-aux-Roses, France.
10 PRES LUNAM, CHU Angers service des Maladies du Sang et INSERM U892, Angers, France.
11 Université Paris 7, Hôpital Saint-Louis, AP-HP, Department of Hematology and Institut Universitaire d'Hématologie, Paris, France.
12 Institut Necker Enfants Malades, INSERM U1151, CNRS UMR 8253, Hôpital Necker-Enfants Malades, Paris, France, and Université Paris Descartes Sorbonne Paris Cité, Paris, France.
13 Department of Clinical Hematology, Hôpital Necker, Assistance publique hôpitaux de Paris, Paris, France.
A. Trinquand, N.R. dos Santos, and C. Tran Quang contributed equally to this article.
Cancer onset and progression involves the accumulation of multiple oncogenic hits, which are thought to dominate or bypass the physiologic regulatory mechanisms in tissue development and homeostasis. We demonstrate in T-cell acute lymphoblastic leukemia (T-ALL) that, irrespective of the complex oncogenic abnormalities underlying tumor progression, experimentally induced, persistent T-cell receptor (TCR) signaling has antileukemic properties and enforces a molecular program resembling thymic negative selection, a major developmental event in normal T-cell development. Using mouse models of T-ALL, we show that induction of TCR signaling by high-affinity self-peptide/MHC or treatment with monoclonal antibodies to the CD3ε chain (anti-CD3) causes massive leukemic cell death. Importantly, anti-CD3 treatment hampered leukemogenesis in mice transplanted with either mouse- or patient-derived T-ALLs. These data provide a strong rationale for targeted therapy based on anti-CD3 treatment of patients with TCR-expressing T-ALL and demonstrate that endogenous developmental checkpoint pathways are amenable to therapeutic intervention in cancer cells.
Cancer Discovery
http://cancerdiscovery.aacrjournals.org/content/6/9/972.abstract




