Associação Portuguesa de Investigação em Cancro
Absence of Telomerase Leads to Immune Response and Tumor Regression in Zebrafish
Absence of Telomerase Leads to Immune Response and Tumor Regression in Zebrafish
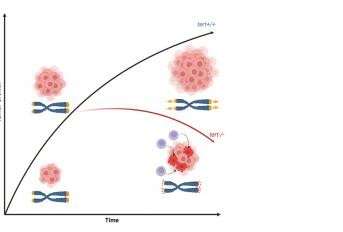
Lopes-Bastos et al. report that melanoma in zebrafish initiates without telomerase (or ALT) activation. However, in late progression, telomerase becomes essential for sustained tumor growth. Tumors that fail to re-activate telomerase slow down growth and even regress. This occurs due to tumor-autonomous (genomic instability) and non-tumor-autonomous (immune response) mechanisms. Highlights • Melanoma initiation and early progression occur without the need for telomerase (or ALT) • Telomerase re-activation is crucial for sustained tumor growth at later stages • Lack of telomerase leads to tumor regression via genome instability and immune response • Nevertheless, telomerase-negative melanomas remain aggressive and lethal.
Bruno Lopes-Bastos1,2, Joana Nabais2, Tânia Ferreira2, Giulia Allavena1, Mounir El Maï1,2, Malia Bird1, Seniye Targen1, Lorenzo Tattini1, Da Kang3, Jia-Xing Yue3, Gianni Litti1, Tânia G. Carvalho4 and Miguel Godinho Ferreira1,2
1- Institute for Research on Cancer and Aging of Nice (IRCAN), CNRS UMR7284, INSERM U1081, Université Côte d’Azur, 06107 Nice, France.
2- Instituto Gulbenkian de Ciência, 2780-156 Oeiras, Portugal.
3- State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou, China.
4- Champalimaud Center for the Unknown, 1400-038 Lisboa, Portugal
Most cancers reactivate telomerase to maintain telomere length and thus acquire immortality. Activating telomerase promoter mutations are found in many cancers, including melanoma. However, it is unclear when and if telomerase is strictly required during tumorigenesis. We combined the telomerase mutant (tert-/-) with two established zebrafish melanoma models. We show that, tert-/- melanomas initially develop with similar incidence and invasiveness as tert+/+ tumors. However, they eventually decline in growth and regress. Late tert-/- tumors exhibit reduced cell proliferation, increased apoptosis, and melanocyte differentiation. Notably, these tumors show enhanced immune cell infiltration and can resume growth when transplanted into immunocompromised hosts. We propose that telomerase is required for melanoma in zebrafish, albeit at later stages of progression, to sustain tumor growth while avoiding immune rejection and regression. Thus, absence of telomerase restricts melanoma through tumor autonomous mechanisms (cell cycle arrest, apoptosis and melanocyte differentiation) and a non-tumor autonomous mechanism (immune rejection).
Cell Reports
https://www.cell.com/cell-reports/fulltext/S2211-1247(24)01386-X




