Associação Portuguesa de Investigação em Cancro
A new study reveals that the RNA‑binding protein YTHDF3 affects gastric cancer cell migration and response to paclitaxel by regulating EZRIN
A new study reveals that the RNA‑binding protein YTHDF3 affects gastric cancer cell migration and response to paclitaxel by regulating EZRIN
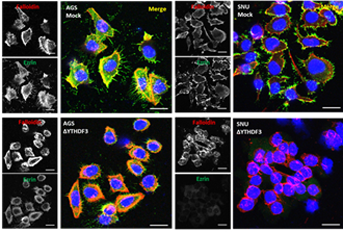
Researchers from the "Differentiation and Cancer" group at i3S, in collaboration with “Cancer Metastasis” and “Cell Division & Genomic Stability” groups and Carmen Jerónimo from IPO-Porto, elucidated the role of YTHDF3, a key m6A RNA “reader” protein, as a driver of gastric cancer (GC) progression and paclitaxel sensitivity. This new study, entitled "The RNA‑binding protein YTHDF3 affects gastric cancer cell migration and response to paclitaxel by regulating EZRIN," was recently published in the journal "Gastric Cancer”. Gastric cancer remains a global health concern, often diagnosed at advanced stages with poor prognosis. By analyzing tumor samples from 331 patients, researchers found that YTHDF3 is significantly overexpressed in GC. Those GC cases overexpressing YTHDF3 benefit more from treatment with chemotherapy. Additionally, CRISPR-Cas9 knockout of YTHDF3 in GC cells impaired migration, metastasis, mitotic orientation, and cytoskeletal organization, while improving paclitaxel sensitivity. Mechanistic data revealed that YTHDF3 regulates EZRIN through m6A RNA modification, forming a YTHDF3–EZR axis that influences tumor behavior, and thereby offering a potential therapeutic strategy.
Authors and Affiliations:
Patrícia Mesquita1,2 , Alexandre Coelho1,3 , Ana S. Ribeiro1,2 , Luís F. C. Póvoas1,4 , Catarina de Oliveira1 , Nelson Leça1,4 , Sara Silva1 , Diana Ferreira1 , Diana Pádua1,3 , Ricardo Coelho6 , Carmen Jerónimo3,7 , Joana Paredes1,2,8 , Carlos Conde1,3,5 , Bruno Pereira1,2 , Raquel Almeida1,2,4
1 i3S ‑ Institute for Research and Innovation in Health, University of Porto, 4200‑135 Porto, Portugal
2 IPATIMUP ‑ Institute of Molecular Pathology and Immunology, University of Porto, 4200‑465 Porto, Portugal
3 ICBAS – School of Medicine and Biomedical Sciences, University of Porto, 4050‑313 Porto, Portugal
4 Biology Department, Faculty of Sciences, University of Porto, 4169‑007 Porto, Portugal
5 IBMC ‑ Institute of Molecular and Cell Biology, University of Porto, 4200‑135 Porto, Portugal
6 Ovarian Cancer Research, Department of Biomedicine, University Hospital Basel and University of Basel, 4031 Basel, Switzerland
7 Cancer Biology and Epigenetics Group, Research Center of IPO Porto (CI‑IPOP)/RISE@CI‑IPOP (Health Research Network), Portuguese Oncology Institute of Porto (IPO Porto)/Porto Comprehensive Cancer Center Raquel Seruca (Porto.CCC Raquel Seruca), 4200‑072 Porto, Portugal
8 Pathology Department, Faculty of Medicine, University of Porto, 4200‑319 Porto, Portugal
Abstract:
Background: Gastric cancer (GC) is the fourth most common cause of cancer-related mortality and the fifth most common cancer worldwide. Despite efforts, the identification of biomarkers and new therapeutic approaches for GC remains elusive. Recent studies have begun to reveal the role of N6-adenosine methylation (m6A) in the regulation of gene expression.
Methods: The expression of the reader YT521-B homology domain-containing family 3 (YTHDF3) in GC was assessed in 331 patients using immunohistochemistry. GC cell lines depleted of YTHDF3 using CRISPR-Cas9 were evaluated for migration, metastasis, orientation of the mitotic spindle, and response to paclitaxel. The association between YTHDF3 and EZRIN (EZR) mRNA was shown using RNA sequencing, immunofluorescence, real-time PCR, and RNA immunoprecipitation. The single-base elongation- and ligation-based qPCR amplification (SELECT) method was used to map m6A in the EZR transcript.
Results: YTHDF3 was significantly overexpressed in GC, and high levels of YTHDF3 were predictive of the response to chemotherapy. In GC cell lines, YTHDF3 was the most highly expressed reader protein. YTHDF3 depletion impaired cytoskeleton organization, cell migration and metastasis, and orientation of the mitotic spindle, leading to an increased response to paclitaxel. EZR was one of the downregulated targets in the YTHDF3 knockout cell models and was associated with the observed phenotype.
Conclusion: YTHDF3 contributes to cell motility and response to paclitaxel in GC cell lines, at least in part through EZR regulation. The YTHDF3-EZR regulatory axis is a novel molecular player in GC, with clinical relevance and potential therapeutic utility.
Journal: Gastric Cancer
Link: https://link.springer.com/article/10.1007/s10120-025-01620-y




