Associação Portuguesa de Investigação em Cancro
Disruption of breast cancer cell pH dynamics decreases aggressiveness and potentiates response to chemotherapy
Disruption of breast cancer cell pH dynamics decreases aggressiveness and potentiates response to chemotherapy
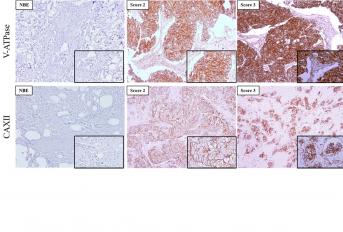
The reverse pH gradient is a common feature of cancer cells, in which the intracellular pH is more alkaline and the extracellular more acidic. This phenotype is supported by increased activity of pH regulators like ATPases, carbonic anhydrases (CAs), monocarboxylate transporters (MCTs) and sodium–proton exchangers (NHEs). In this work, we analyzed the expression of these pH regulators and explored their inhibition in breast cancer cells as a therapeutic strategy. The different pH regulators were overexpressed at the plasma membrane of malignant cells, when compared to non-tumoral tissue and their expression varied with the cancer subtype. pH regulator inhibition decreased cancer cell aggressiveness and sensitized the cells to the effect of doxorubicin (chemotherapy), meaning that lower doses of chemotherapy are necessary, decreasing the associated adverse effects. Our results support proton dynamic disruption as a breast cancer anticancer strategy, either alone or combined with chemotherapy.
Authors and Affiliations:
Diana Tavares-Valente1,2,3, Bárbara Sousa4,5, Fernando Schmitt4,6, Fátima Baltazar1,2 and Odília Queirós3
1 Life and Health Sciences Research Institute (ICVS), School of Medicine, University of Minho, 4710-057 Braga, Portugal;
2 PT Government Associate Laboratory, ICVS/3B’s—Life and Health Sciences Research Institute/Biomaterials, Biodegradables and Biomimetics, 4710-057 Braga/Guimarães, Portugal
3 Department of Sciences, IINFACTS—Institute of Research and Advanced Training in Health Sciences and Technologies, University Institute of Health Sciences (IUCS), CESPU, CRL, 4585-116 Gandra, Portugal
4 IPATIMUP—Institute of Molecular Pathology and Immunology of the University of Porto, University of Porto, 4200-135 Porto, Portugal;
5 i3S—Institute for Research & Innovation in Health, University of Porto, 4200-135 Porto, Portugal
6 FMUP—Faculty of Medicine of the University of Porto, University of Porto, 4200-319 Porto, Portugal
Abstract:
The reverse pH gradient is a major feature associated with cancer cell reprogrammed metabolism. This phenotype is supported by increased activity of pH regulators like ATPases, carbonic anhydrases (CAs), monocarboxylate transporters (MCTs) and sodium–proton exchangers (NHEs) that induce an acidic tumor microenvironment, responsible for the cancer acid-resistant phenotype. In this work, we analyzed the expression of these pH regulators and explored their inhibition in breast cancer cells as a strategy to enhance the sensitivity to chemotherapy. Expression of the different pH regulators was evaluated by immunofluorescence and Western blot in two breast cancer cell lines (MDA-MB-231 and MCF-7) and by immunohistochemistry in human breast cancer tissues. Cell viability, migration and invasion were evaluated upon exposure to the pH regulator inhibitors (PRIs) concanamycin-A, cariporide, acetazolamide and cyano-4 hydroxycinnamate. Additionally, PRIs were combined with doxorubicin to analyze the effect of cell pH dynamic disruption on doxorubicin sensitivity. Both cancer cell lines expressed all pH regulators, except for MCT1 and CAXII, only expressed in MCF-7 cells. There was higher plasma membrane expression of the pH regulators in human breast cancer tissues than in normal breast epithelium. Additionally, pH regulator expression was significantly associated with different molecular subtypes of breast cancer. pH regulator inhibition decreased cancer cell aggressiveness, with a higher effect in MDA-MB-231. A synergistic inhibitory effect was observed when PRIs were combined with doxorubicin in the breast cancer cell line viability. Our results support proton dynamic disruption as a breast cancer antitumor strategy and the use of PRIs to boost the activity of conventional therapy.
Journal: Pharmaceutics
Link: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7916175/pdf/pharmaceutics-13-00242.pdf




