Associação Portuguesa de Investigação em Cancro
RPSA was identified for the first time as a hydrogen peroxide sensor that regulates adhesion of tumor cells
RPSA was identified for the first time as a hydrogen peroxide sensor that regulates adhesion of tumor cells
Hydrogen peroxide (H2O2) is a well-known antiseptic used in our homes since the 1920s. But did you know that our cells produce this molecule and that at low concentrations H2O2 instructs our cells to do different things, acting as a signaling molecule? In fact, H2O2 may oxidize several cellular components including proteins, altering their function. Healthy cells use H2O2 to regulate several of their behaviors, including proliferation, migration, or even cell-to-cell communication. It is, therefore, not surprising that cancer cells produce higher amounts of this reactive oxygen species and take advantage of this signaling feature of H2O2 to increase their proliferation and migration,.
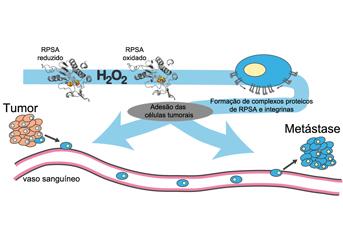
Although the knowledge around this subject has been increasing in the past few years, it is still largely unknown which molecules are oxidized by H2O2 and how this oxidation affects their function. In our recent work, we have found that a protein called Ribosomal Protein SA (RPSA) is oxidized by H2O2 and that this oxidation enhances cancer properties. In fact, RPSA oxidation improves cell adhesion efficiency to the extracellular matrix and promotes cell extravasation (the passing of cells from the blood stream to surrounding tissues, a critical step in the metastatic process). The effect on cell adhesion is most likely mediated by RPSA clusters that accumulate at the cell membrane level and contain specific adhesion molecules called integrins. Interestingly, it appears that different adhesion molecules are present on those clusters depending on the RPSA redox state, suggesting that RPSA oxidation may influence the type of extracellular matrix to which cells adhere.
Our results unravel a new mechanism by which H2O2 modulates the cell adhesion properties and identify RPSA as the H2O2 sensor in this process. Elevated levels of H2O2 produced both in primary tumors and secondary metastatic sites, together with RPSA up-regulation, might confer a selective advantage to tumor cells in the metastatic process by contributing to alterations in cell adhesion properties such as binding specificity. The identification of redox-regulated proteins, such as RPSA and the determination of their relevance in tumor development will be essential to design more specific and efficient redox-based therapies for cancer.
Authors and Afilliations:
Filipe Vilas-Boasa, Ana Bagulhoa, Rita Tenentea, 2, Vitor H. Teixeiraa, Gabriel G. Martinsb, c, Gonçalo da Costaa, Ana Jerónimoa, Carlos Cordeiroa, Miguel Machuqueiroa, Carla Reala
a Centro de Química e Bioquímica, Faculdade de Ciências, Universidade de Lisboa, 1749-016 Lisboa, Portugal
b Instituto Gulbenkian de Ciência, R. Quinta Grande 6, 2780-156 Oeiras, Portugal
c CE3C – Centre for Ecology, Evolution and Environmental Changes, Faculdade de Ciências, Universidade de Lisboa, Campo Grande, 1749-016 Lisboa, Portugal
2 Instituto de Investigação Científica Tropical, 1349-007 Lisboa, Portugal
Abstract:
To become metastatic, a tumor cell must acquire new adhesion properties that allow migration into the surrounding connective tissue, transmigration across endothelial cells to reach the blood stream and, at the site of metastasis, adhesion to endothelial cells and transmigration to colonize a new tissue. Hydrogen peroxide (H2O2) is a redox signaling molecule produced in tumor cell microenvironment with high relevance for tumor development. However, the molecular mechanisms regulated by H2O2 in tumor cells are still poorly known. The identification of H2O2-target proteins in tumor cells and the understanding of their role in tumor cell adhesion are essential for the development of novel redox-based therapies for cancer.
In this paper, we identified Ribosomal Protein SA (RPSA) as a target of H2O2 and showed that RPSA in the oxidized state accumulates in clusters that contain specific adhesion molecules. Furthermore, we showed that RPSA oxidation improves cell adhesion efficiency to laminin in vitro and promotes cell extravasation in vivo.
Our results unravel a new mechanism for H2O2-dependent modulation of cell adhesion properties and identify RPSA as the H2O2 sensor in this process. This work indicates that high levels of RPSA expression might confer a selective advantage to tumor cells in an oxidative environment.
Journal: Free Radical Biology & Medicine
Link: http://www.sciencedirect.com/science/article/pii/S089158491501117X




