Associação Portuguesa de Investigação em Cancro
New epigenetic biomarkers for the detection of bladder cancer in urine
New epigenetic biomarkers for the detection of bladder cancer in urine
Sara Monteiro-Reis (1), Ana Blanca (2), Joana Tedim-Moreira (1), Isa Carneiro (1,3), Diana Montezuma (1,3), Paula Monteiro (1,3), Jorge Oliveira (4), Luís Antunes (5), Rui Henrique (1,2,6), António Lopez-Beltran (7,8) e Carmen Jerónimo (1,6)
(1) Grupo de Epigenética e Biologia do Cancro – Centro de Investigação (CI-IPOP), Instituto Português de Oncologia do Porto Francisco Gentil EPE (IPO Porto), e Porto Comprehensive Cancer Center (P.CCC), Porto, Portugal;
(2) Servicio de Urología, Hospital Universitario Reina Sofía, Instituto Maimónides de Investigación Biomédica de Córdoba, Espanha;
(3) Serviço de Anatomia Patológica, IPO-Porto;
(4) Clínica de Urologia, IPO-Porto;
(5) Serviço de Epidemiologia, IPO Porto & Grupo de Epidemiologia do Cancro – CI-IPOP;
(6) Departamento de Imunologia e Patologia Molecular, Instituto de Ciência Biomédicas Abel Salazar (ICBAS) – Universidade do Porto, Portugal; (7) Departamento Anatomía Patológica, Facultad de Medicina, Universidad de Córdoba, Espanha;
(8) Centro Clínico Champalimaud, Lisboa, Portugal.
Abstract:
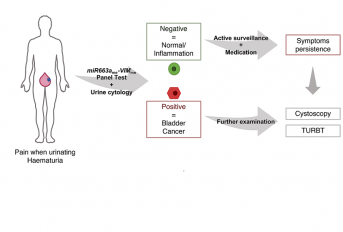
Bladder cancer (BlCa) is a common malignancy with significant morbidity and mortality. Current diagnostic methods are invasive and costly, showing the need for newer biomarkers. Although several epigenetic-based biomarkers have been proposed, their ability to discriminate BlCa from common benign conditions of the urinary tract, especially inflammatory diseases, has not been adequately explored. Herein, we sought to determine whether VIMme and miR663ame might accurately discriminate those two conditions, using a multiplex test. Performance of VIMme and miR663ame in tissue samples and urines in testing set confirmed previous results (96.3% sensitivity, 88.2% specificity, area under de curve (AUC) 0.98 and 92.6% sensitivity, 75% specificity, AUC 0.83, respectively). In the validation sets, VIMme-miR663ame multiplex test in urine discriminated BlCa patients from healthy donors or patients with inflammatory conditions, with 87% sensitivity, 86% specificity and 80% sensitivity, 75% specificity, respectively. Furthermore, positive likelihood ratio (LR) of 2.41 and negative LR of 0.21 were also disclosed. Compared to urinary cytology, VIMme-miR663ame multiplex panel correctly detected 87% of the analysed cases, whereas cytology only forecasted 41%. Furthermore, high miR663ame independently predicted worse clinical outcome, especially in patients with invasive BlCa. We concluded that the implementation of this panel might better stratify patients for confirmatory, invasive examinations, ultimately improving the cost-effectiveness of BlCa diagnosis and management. Moreover, miR663ame analysis might provide relevant information for patient monitoring, identifying patients at higher risk for cancer progression.
Journal of Clinical Medicine
https://www.mdpi.com/2077-0383/9/2/605




